At present, common thyroid hormone detection methods include Chemiluminescent Immunoassay (CLIA) and Isotope Dilution Liquid Chromatography Tandem Mass Spectrometry (ID-LC /MS /MS). The accuracy of the LC-MS/MS results is clinically recognized, but why is CLIA still the most preferred method for thyroid hormone detection?
The difficulty of the application of LC-MS/MS in clinical thyroid hormones detection mainly lies in the fT3/fT4 detection, in which, the separation of fT3/fT4 needs to ensure the endogenous balance between the bound state and the free state. Current LC-MS/MS separation system takes a processing time of 30 min-24h, which is very time-consuming. Moreover, it is a challenge to avoid non-specific binding of the sample to the surface of the membrane or device during the separation process.
In conclusion, the complex and time-consuming sample pre-processing for LC-MS/MS, the unavoidable non-specific binding on the membrane surface, and the demands for automation in clinics have made the popularization of LC-MS/MS in clinical testing particularly difficult.
T3/T4 Sandwich Antibody
High Sensitivity · One Step Assay · ICA/CLIA Verified
The binding sites of small molecule compounds such as T3 and T4 and the corresponding antibodies are mostly in a cave structure, and most of their structure is covered, resulting in insufficient space for antibody binding. Therefore, traditional Sandwich Immunoassay technology is difficult to detect small molecules, and most of the T3/T4 detection kits use Competitive Immunoassay. With years of experience in developing small molecule sandwich antibodies, OkayBio has successfully developed T3 and T4 sandwich antibodies.
OkayBio T3 sandwich antibody enables the CLIA detection to realize the upgrade from two-step Competitive Immunoassay to One-step Sandwich Immunoassay, which reduces 50% of the consuming time for thyroid function tests. It is a tremendous breakthrough for multi-projects combined thyroid function tests and POCT rapid tests.
Advantage of Sandwich Immunoassay

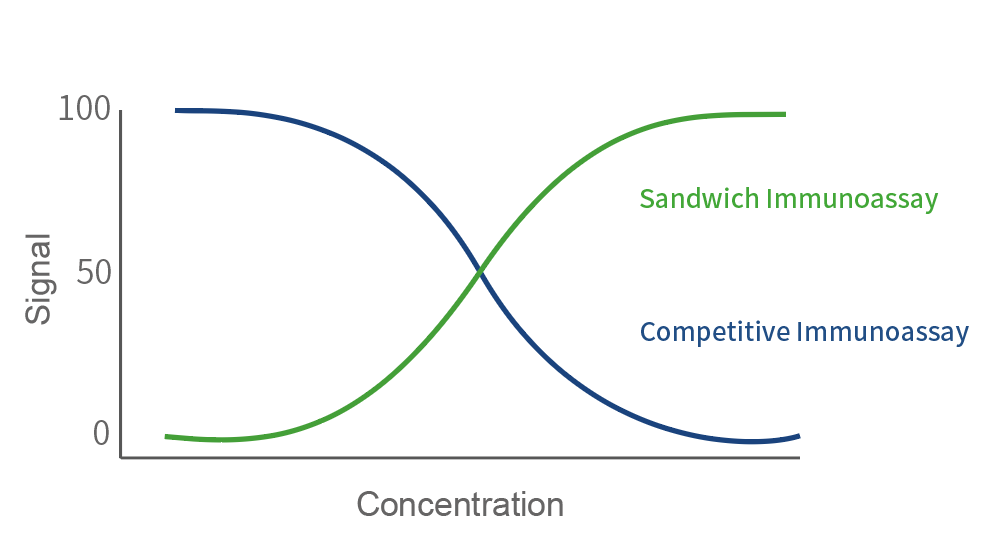
Figure 1. Difference in signal: Sandwich VS Competitive
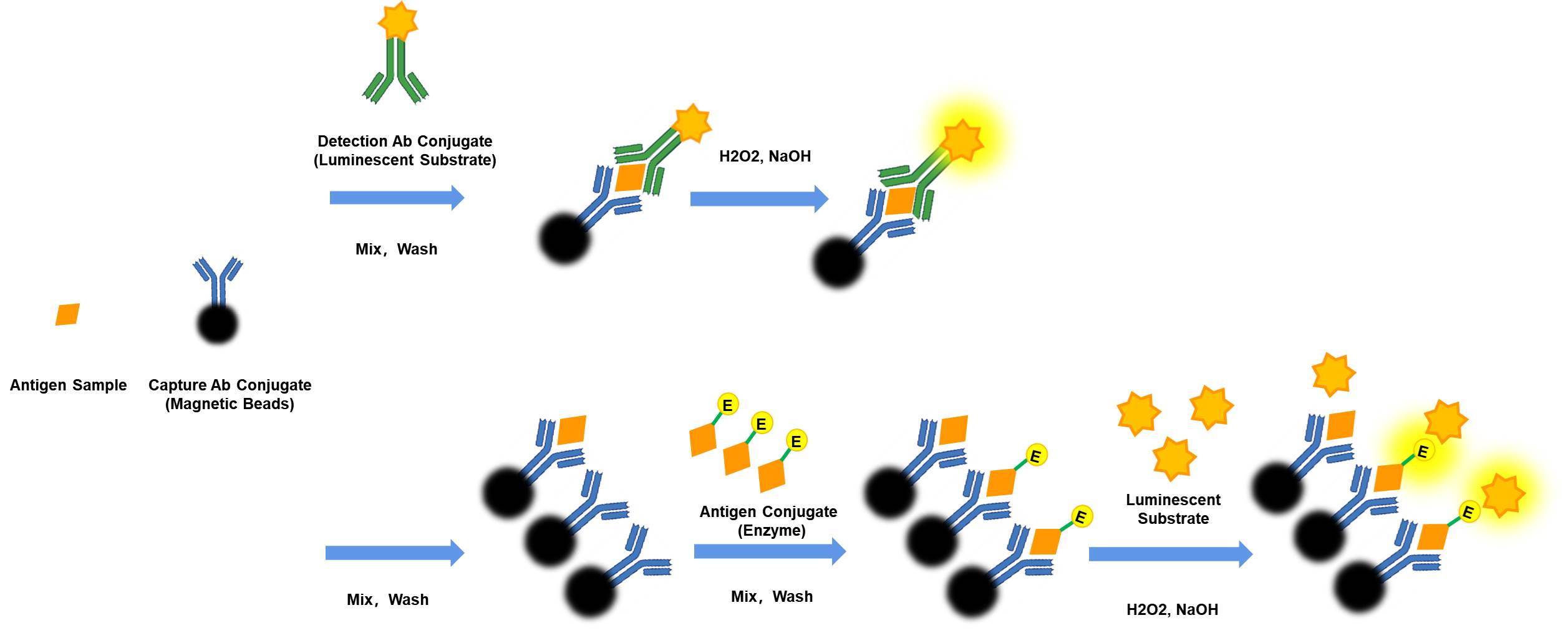
Figure 2. Work flow in Chemiluminescent immunoassay (CLIA) Sandwich VS Competitive: The Sandwich Immunoassay facilitates a streamlined "One-step assay" approach, wherein the capture monoclonal antibody (mAb), detection mAb, and the sample can be mixed or incubated simultaneously. This innovative process results in a significant time-saving of 50% compared to the traditional Competitive Immunoassay, where separate incubation and washing steps are mandatory for the antibody-antigen interaction.
T3 Monoclonal Antibody
Product Performance
T3 sandwich antibody pair (R515n5-R514n5) has been verified with its signal-to-noise ratio (S/N) on the direct Chemiluminescent Immunoassay (CLIA) platform is 10 times that of the Competitive Immunoassay. The sensitivity of fT3 detection is ≥0.1pmol/L, and the correlation between the detection results and Roche assigned samples was R2 >0.99 on Chemiluminescent Immunoassay (CLIA) and the correlation of T3 sandwich antibody pair (R513c8, R515n5) was R2 >0.94 on Time-resolved Fluorescent Immunoassay (TRFIA).
Chemiluminescent Immunoassay (CLIA) platform
Roche assigned fT3/tT3 clinical samples were tested via the R515n5 (Capture)- R514n5 (Detection) pair on direct Chemiluminescent Immunoassay (CLIA) platform.
fT3 Lowest Detection Limit
Direct Chemiluminescent Immunoassay (CLIA)
| Calibrator |
Concentration(pmol/L) |
RLU avg. (20 times) |
Curve |
| S1 |
0 |
5549 |
y=38133x+5407 |
| S2 |
3.3 |
131246 |
| SD |
143.2 |
| Lowest Detection Limit (pmol/L) |
0.011 |
fT3 Sample Coincidence Rate
Direct Chemiluminescent Immunoassay (CLIA)
| Sample |
Concentration
(pmol/L) |
RLU |
| 1 |
0.000 |
5407 |
| 2 |
3.32 |
131246 |
| 3 |
7.15 |
265489 |
| 4 |
18.63 |
856983 |
| 5 |
30.12 |
1656988 |
| 6 |
49.55 |
2556942 |
Table 1. fT3 Clinical comparison analysis data on CLIA

Figure 3. fT3 Standard curve on CLIA
tT3 Sample Coincidence Rate
Direct Chemiluminescent Immunoassay (CLIA)
| 序号 |
浓度值
(nmol/L) |
RLU |
| 1 |
0.000 |
3407 |
| 2 |
0.40 |
90705 |
| 3 |
1.00 |
244456 |
| 4 |
2.48 |
1094240 |
| 5 |
4.94 |
3139387 |
| 6 |
10.12 |
7028859 |
Table 2. tT3 Clinical comparison analysis data on CLIA

Figure 4. tT3 Standard curve on CLIA
Cross Reactivity
Direct Chemiluminescent Immunoassay (CLIA)
L-T4 at 500ng/mL was measured via T4 sandwich antibodies (R515n5, R514n5), and the cross-reactivity was 0.01%.
Time-resolved Fluorescent Immunoassay (TRFIA) platform
Roche assigned fT3/tT3 clinical samples were tested via the R513c8 (Capture) - R515n5 (Detection) antibody pair on Time-resolved Fluorescent Immunoassay (TRFIA) platform.
f/tT3 Sample Coincidence Rate
Time-resolved Fluorescent Immunoassay (TRFIA)

Figure 5. fT3 Standard curve on TRFIA

Figure 6. tT3 Standard curve on TRFIA
Concentration
(ng/mL) |
T value |
C value |
T/C |
| 0 |
205 |
20862 |
0.0098 |
| 0.08 |
5411 |
20484 |
0.2641 |
| 0.16 |
10342 |
21160 |
0.4888 |
| 0.8 |
40969 |
19130 |
2.1416 |
| 4 |
64041 |
19371 |
3.3061 |
| 20 |
84362 |
21193 |
3.9807 |
Table 3. fT3 Clinical comparison analysis data on TRFIA
Concentration
(ng/mL) |
T value |
C value |
T/C |
| 5 |
345 |
22103 |
0.0156 |
| 20 |
1564 |
20159 |
0.0776 |
| 100 |
8298 |
18947 |
0.4380 |
| 500 |
31267 |
21537 |
1.4518 |
| 2000 |
50473 |
20476 |
2.4650 |
Table 4. tT3 Clinical comparison analysis data on TRFIA
T4 Monoclonal Antibody
Product Performance
T4 sandwich antibody pair (R555a5-R534a7) has been verified with its correlation between the detection results and Roche assigned samples R2 >0.96 on the direct Chemiluminescent Immunoassay (CLIA) platform. R533c3-R555a5 pair has been verified with correlation R2 >0.94 on the Time-resolved Fluorescent Immunoassay (TRFIA) platform.
Chemiluminescent Immunoassay (CLIA) platform
Roche assigned fT4/tT4 clinical samples were tested via the R555a5 (Capture)- R534a7 (Detection) pair on direct Chemiluminescent Immunoassay (CLIA) platform.
fT4 Sample Coincidence Rate
Direct Chemiluminescent Immunoassay (CLIA)
| Sample |
Concentration
(pmol/L) |
RLU |
| 1 |
0.000 |
3384 |
| 2 |
4.63 |
225668 |
| 3 |
11.38 |
516982 |
| 4 |
19.25 |
865801 |
| 5 |
52.25 |
2376942 |
| 6 |
102.50 |
4750644 |
Table 5. fT4 Clinical comparison analysis data on CLIA

Figure 7. fT4 Standard curve on CLIA
tT4 Sample Coincidence Rate
Direct Chemiluminescent Immunoassay (CLIA)
| Sample |
Concentration
(pmol/L) |
RLU |
| 1 |
0.000 |
3055 |
| 2 |
20.86 |
229219 |
| 3 |
41.65 |
422718 |
| 4 |
85.96 |
893682 |
| 5 |
120.12 |
1233027 |
| 6 |
302.40 |
3039924 |
Table 6. tT4 Clinical comparison analysis data on CLIA

Figure 8. tT4 Standard curve on CLIA
Cross Reactivity
Direct Chemiluminescent Immunoassay (CLIA)
L-T3 at 10000ng/mL was measured via T4 sandwich antibodies (R555a5-R534a7), and the cross-reactivity was 0%.
Time-resolved Fluorescent Immunoassay (TRFIA) platform
Roche assigned fT4/tT4 clinical samples were tested via the R533c3(Capture)-R555a5(Detection) antibody pair on Time-resolved Fluorescent Immunoassay (TRFIA) platform.
f/tT3 Sample Coincidence Rate
Time-resolved Fluorescent Immunoassay (TRFIA)

Figure 9. fT4 Standard curve on TRFIA

Figure 10. tT4 Standard curve on TRFIA
Related Products
| Product Name |
Catalog# |
Recommend Pair |
Platform |
| T3 Antibody |
R515n5 |
R515n5(Capture)-R514n5(Detection)
R515n5(Capture)-R513c8(Detection) |
CLIA |
| R514n5 |
| R513c8 |
R513c8(Capture)-R515n5(Detection) |
ICA |
| T4 Antibody |
R533c3 |
R533c3(Capture)-R555a5(Detection) |
CLIA |
| R534a7 |
R555a5(Capture)-R533c3(Detection)
R555a5(Capture)-R534a7(Detection) |
ICA |
| R555a5 |



